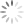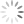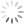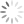P and N Type
N-type Semiconductor
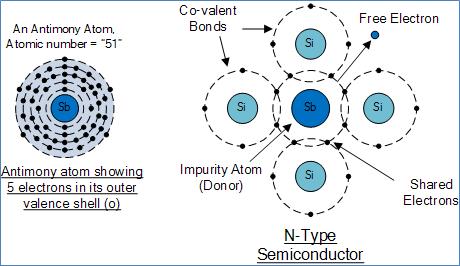
N-Type semiconductor is formed by adding impurity in atoms such as Arsenic, Antimony or Phosphorus into the crystalline structure making it extrinsic. These atoms have five outer electrons in their outermost shell to share with neighboring atoms so they are term as “Pentavalent” impurities. Out of five four orbital electrons are bond with its neighboring silicon atoms leaving one “free electron” to become mobile when an electrical voltage is applied (electron flow). As each impurity atom “donates” one electron, pentavalent atoms are also termed as “donor impurity”. The resulting semiconductor formed after doping material has an excess of current-carrying electrons, each with a negative charge, and is, therefore, term as an N-type material in which electrons called “Majority Carriers” and holes are called “Minority Carriers”.
Antimony Atom and Doping
P-Type Semiconductor
P-Type Semiconductor is formed by adding “Trivalent” (3-electron) impurity into the crystalline structure, such as Aluminum, Boron or Indium having three valence electrons available in their outermost shell, so result of this fourth closed bond cannot be formed. And a complete connection and bond are not possible, resulting in semiconductor material an excess of positively charged carriers known as holes in the structure of the crystal where electrons are less in number and missing. Trivalent impurities are generally known as “Acceptors” as they are continually “accepting” extra or free electrons.
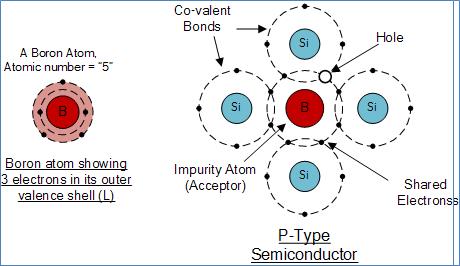
Boron (symbol B) is generally used as a trivalent impurity as it has only five electrons arranged in three shells around its nucleus with the outermost orbital having only three electrons. The doping of Boron atoms creates positive charge carriers resulting in a P-type material with the positive holes being called “Majority Carriers” while the free electrons are called “Minority Carriers”.
Boron Atom and Doping